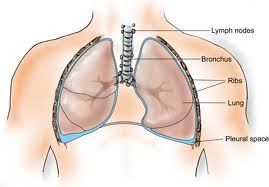
After nearly a century of mesothelioma analysis, doctors have learned what causes this cancer, who is most in danger for contracting the disease, what symptoms indicate its presence and what tools are simplest at diagnosing and treating the cancer. These strides have considerably impacted how medical professionals diagnose and treat mesothelioma. Current efforts are operating to develop more practical treatments and an eventual cure for this life-threatening disease.
Need to recognize additional regarding Mesothelioma?
Asbestos.com offers a complimentary packet with data on mesothelioma analysis. Fill out the shape to the correct to possess one delivered to you overnight.
Pharmaceutical Breakthroughs for Mesothelioma
Currently, the FDA has solely approved one chemotherapy medication for the treatment of mesothelioma. Alimta, that works to prevent cell division, is commonly administered together with the platinum-based chemotherapy agent Cisplatin in one in all the foremost effective chemotherapy mixtures obtainable.
Onconase, one in all the primary stem cell medications to succeed in the ultimate stages of clinical trials, may be a low-toxicity chemotherapy drug that's designed to shrink and kill mesothelioma tumors. This medication has been admitted to the FDA’s quick Track program, that expedites the approval time for sure medication. This program usually cuts the approval time to 6 months, permitting the medication in question to be quickly designated for widespread use when patients have few different FDA-approved choices.
Veglin, that has demonstrated ability to stabilize and shrink tumors, reached section II clinical trial testing in 2004. The University of California’s Keck college of medication is currently examining its success in mesothelioma patients. Researchers hope to curb the fast metastasis typical of mesothelioma with this medication.
The process of introducing a replacement mesothelioma medication generally takes between twelve and fifteen years. to make sure patient safety, adequate time should be spent in every stage of the event method. The table below outlines the common method of testing and presenting a replacement pharmaceutical product.
Stage of the method Step of Development
Intensity Modulated Radiation Therapy may be a additional precise sort of delivering radiation to an affected space, in flip sparing the healthy tissues round the tumor. currently implemented in nationwide hospitals, the comparatively new procedure is one in all the foremost precise sorts of externally-delivered radiation therapy.
Gene therapy, that uses laboratory-modified viruses that activate the immune system and kill cancerous cells, continues to be within the experimental section. though not however widespread, clinical trials have yielded promising results. Clinical trials are exploring the effectiveness of photodynamic therapy, that exposes sensitive cancerous cells to light-weight which will kill them, and immunotherapy, a way that manipulates the patient’s immune system into attacking the antigens in cancerous cells.
Biomarkers are generating considerable interest in analysis comes. Identifying specific compounds in fluid or tissue samples will indicate the presence or absence of a precise disease. Fujirebio Diagnostics’ check is that the initial of its kind which will detect the biomarkers related to mesothelioma with an easy blood screen, and its developers hope it'll increase the speed of early diagnosis.
Mesothelioma Clinical Trials
Clinical trials are one in all the foremost ways in which researchers will gather data a couple of specific drug or procedure’s have an effect on on mesothelioma patients. Since the illness is sort of rare, any chance to look at a patient’s response to an emergent treatment will greatly assist medical researchers in understanding the impact of the therapy in question.
Because the aim of clinical trials is to any explore treatments that have illustrated success and safety during a laboratory, participants have the chance to profit from a drug or therapy routine that's not however obtainable through their doctor or oncologist. These trials are professionally monitored, and therefore the nature of the treatment furthermore as its risks and edges are explained to a patient before they offer their informed consent to participate.
Clinical trials involve four phases, every specializing in a vital side of product development.
section I determines basic data like drug dosage or strategies of administration.
section II focuses on the treatment’s safety and interaction with the meant target.
section III compares the new technique of treatment to current choices. If it seems to considerably impact prognosis, FDA approval are going to be requested, which might take up to a year.
section IV begins once the drug has been approved to be used and it becomes clinically accepted. This monitoring is employed to live its continued affects on a wider population.
Mesothelioma analysis Funding
Advocacy teams have brought mesothelioma out of relative obscurity and have even secured federal funding for eligible studies. In 2008, the U.S. Department of Defense allotted $50 million for mesothelioma analysis, and therefore the funds are being distributed in increments of up to $2.5 million.
The Meso Foundation's Science Advisory Board reviews dozens of international analysis proposals every year and awards grants of up to $50,000 per project per year. variety of smaller grants, personal donations and proceeds from freelance fundraisers are awarded to different analysis organizations.
Applying Research: Education and Empowerment
Since 2004, the Annual Asbestos Awareness Conference has been sharing the recent analysis with medical professionals, patients and their families. The Meso Foundation sponsors a yearly International Symposium on Malignant Mesothelioma, and therefore the International Mesothelioma Interest cluster has hosted a yearly mesothelioma conference since 2000. These events, together with smaller native shows hosted by hospitals or physicians, are generally open to the general public and supply attendees with breaking data on mesothelioma treatment, diagnosis and screening analysis.
Need to recognize additional regarding Mesothelioma?
Asbestos.com offers a complimentary packet with data on mesothelioma analysis. Fill out the shape to the correct to possess one delivered to you overnight.
Pharmaceutical Breakthroughs for Mesothelioma
Currently, the FDA has solely approved one chemotherapy medication for the treatment of mesothelioma. Alimta, that works to prevent cell division, is commonly administered together with the platinum-based chemotherapy agent Cisplatin in one in all the foremost effective chemotherapy mixtures obtainable.
Onconase, one in all the primary stem cell medications to succeed in the ultimate stages of clinical trials, may be a low-toxicity chemotherapy drug that's designed to shrink and kill mesothelioma tumors. This medication has been admitted to the FDA’s quick Track program, that expedites the approval time for sure medication. This program usually cuts the approval time to 6 months, permitting the medication in question to be quickly designated for widespread use when patients have few different FDA-approved choices.
Veglin, that has demonstrated ability to stabilize and shrink tumors, reached section II clinical trial testing in 2004. The University of California’s Keck college of medication is currently examining its success in mesothelioma patients. Researchers hope to curb the fast metastasis typical of mesothelioma with this medication.
The process of introducing a replacement mesothelioma medication generally takes between twelve and fifteen years. to make sure patient safety, adequate time should be spent in every stage of the event method. The table below outlines the common method of testing and presenting a replacement pharmaceutical product.
Stage of the method Step of Development
- Years 1-3 Basic analysis
- Years 4-6 Pre-clinical testing using in vitro (artificially created environments) and animal trials
- Years 7-10 Clinical testing via section I, II and III clinical trials
- Year ten Register the drug with the U.S. Food and Drug Administration
- Year eleven Introduction of the drug to the general public
- Years 11-15 Product monitoring and section IV clinical trials
- Novel Procedures
Intensity Modulated Radiation Therapy may be a additional precise sort of delivering radiation to an affected space, in flip sparing the healthy tissues round the tumor. currently implemented in nationwide hospitals, the comparatively new procedure is one in all the foremost precise sorts of externally-delivered radiation therapy.
Gene therapy, that uses laboratory-modified viruses that activate the immune system and kill cancerous cells, continues to be within the experimental section. though not however widespread, clinical trials have yielded promising results. Clinical trials are exploring the effectiveness of photodynamic therapy, that exposes sensitive cancerous cells to light-weight which will kill them, and immunotherapy, a way that manipulates the patient’s immune system into attacking the antigens in cancerous cells.
Biomarkers are generating considerable interest in analysis comes. Identifying specific compounds in fluid or tissue samples will indicate the presence or absence of a precise disease. Fujirebio Diagnostics’ check is that the initial of its kind which will detect the biomarkers related to mesothelioma with an easy blood screen, and its developers hope it'll increase the speed of early diagnosis.
Mesothelioma Clinical Trials
Clinical trials are one in all the foremost ways in which researchers will gather data a couple of specific drug or procedure’s have an effect on on mesothelioma patients. Since the illness is sort of rare, any chance to look at a patient’s response to an emergent treatment will greatly assist medical researchers in understanding the impact of the therapy in question.
Because the aim of clinical trials is to any explore treatments that have illustrated success and safety during a laboratory, participants have the chance to profit from a drug or therapy routine that's not however obtainable through their doctor or oncologist. These trials are professionally monitored, and therefore the nature of the treatment furthermore as its risks and edges are explained to a patient before they offer their informed consent to participate.
Clinical trials involve four phases, every specializing in a vital side of product development.
section I determines basic data like drug dosage or strategies of administration.
section II focuses on the treatment’s safety and interaction with the meant target.
section III compares the new technique of treatment to current choices. If it seems to considerably impact prognosis, FDA approval are going to be requested, which might take up to a year.
section IV begins once the drug has been approved to be used and it becomes clinically accepted. This monitoring is employed to live its continued affects on a wider population.
Mesothelioma analysis Funding
Advocacy teams have brought mesothelioma out of relative obscurity and have even secured federal funding for eligible studies. In 2008, the U.S. Department of Defense allotted $50 million for mesothelioma analysis, and therefore the funds are being distributed in increments of up to $2.5 million.
The Meso Foundation's Science Advisory Board reviews dozens of international analysis proposals every year and awards grants of up to $50,000 per project per year. variety of smaller grants, personal donations and proceeds from freelance fundraisers are awarded to different analysis organizations.
Applying Research: Education and Empowerment
Since 2004, the Annual Asbestos Awareness Conference has been sharing the recent analysis with medical professionals, patients and their families. The Meso Foundation sponsors a yearly International Symposium on Malignant Mesothelioma, and therefore the International Mesothelioma Interest cluster has hosted a yearly mesothelioma conference since 2000. These events, together with smaller native shows hosted by hospitals or physicians, are generally open to the general public and supply attendees with breaking data on mesothelioma treatment, diagnosis and screening analysis.